Nanobiology research by world-leading AFM technology
Biophysics Lab, School of Mathematics and Physics, Institute of Science and Engineering Professor Toshio Ando
Directly observing protein dynamics
Leading life science by research on nanometer world
Proteins, which are formed by linear linkage of twenty kinds of amino acids, are important molecules essential to all organisms. They are very small. The size ranges from several nanometers to several tens of nanometers. There are several tens of thousands kinds of proteins in the human body. Each of them has a specific function.
For example, the protein called myosin that exists in heart, skeletal, and other muscles brings about muscle contraction by interacting with a protein called actin and hydrolyzing ATP molecules, which serve as an energy source. When you move your arms and legs, these proteins are working. Even our memory and thinking rely on the action of proteins such as channel and receptor proteins that are present in neurons.
High-speed AFM capable of capturing movement of proteins on video
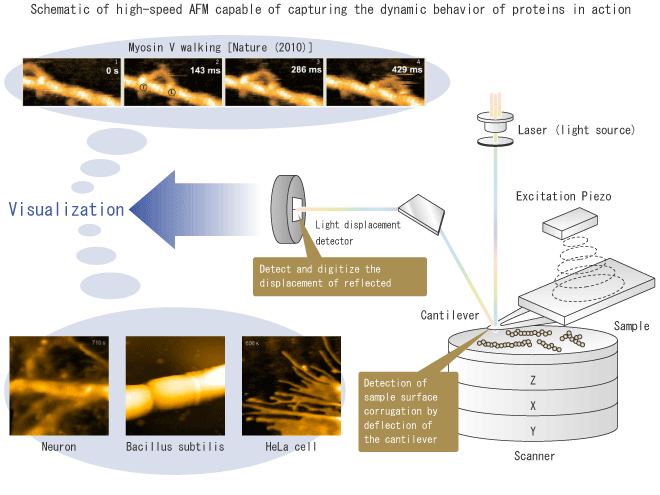
In our research project, we aim to elucidate the functional mechanism of biomolecules including proteins by their dynamic visualization. We have developed the world's most advanced microscope, the high-speed AFM (atomic force microscope) to directly observe moving molecules. The microscope, AFM, can observe the three-dimensional structure of sample surfaces at the atomic level. It is called the atomic force microscope because it detects the force exerting between atoms on the probe and sample surfaces. Since samples in various environments (vacuum, air, and liquids) can be observed under nearly natural conditions, it is widely considered as a leading edge nanoscale analytical tool for life sciences (and other sciences).
We succeeded in markedly improving the speed performance of AFM, spending about fifteen years. We can now capture images at the maximum rate of 33 frames per second. We call this microscope "high-speed AFM". We also minimized the force with which the probe touches the sample, to make it possible to observe the action of proteins without disturbing them.
Currently, we are studying in two directions: 1) Understanding the functional mechanism of more proteins. This has been pursued by the collaboration with intra- and external research communities. 2) Attempting to develop new conceptual microscopes that allow observing the dynamic molecular processes that occur on the surfaces of live cells and intracellular organelles.
If achieve 2), we will be able to directly observe, for example, the dynamic processes occurring in the synapses of neurons that are closely related to memory and learning, and the dynamic processes by which proteins are transported into the interior of mitochondria.
Seeking further observation of dynamic action of proteins
With the development of high-speed AFM, we are now able to directly observe the movement of proteins molecules in aqueous solution as motion pictures. Before the advent of high-speed AFM, the dynamic action of molecules had to be inferred.
One of our achievements is that we successfully captured walking myosin V on video. When myosin V transports melanin pigments or neurotrasmitters within cells, it moves along actin filaments with a thin fibrous structure. Its step size is approximately 36 nm (nanometers), and compared it to human this corresponds to running at a speed of 100 m in 10.28 seconds. The high-speed AFM observation revealed that the leading leg of a myosin V molecule rotates forward upon trailing leg detachment from actin. Moreover, we elucidated the walking mechanism as well as the mechanism of coupling between ATP hydrolysis and the mechanical movement of myosin V.
To further expand our world-first achievement of filming protein molecules in action, we have been working to commercialize the high-speed AFM instrument and provided it to several laboratories around the world. We would like to contribute to the elucidation of functional mechanism of many different proteins by having many researchers use this new tool.
Our ultimate goal is to make full use of this leading edge microscopy, to create a new field in life sciences, and to make Kanazawa University a unique and strong place to study science in the new field.